FOR RELEASE: Monday, March 22, 1999, 15:27 EST
Daniel P. Aalberts
(413-597-3520,
aalberts@williams.edu),
Williams College
Williamstown, MA 01267 USA
Local phone in Atlanta: 404-521-0000 (3/22/99 - 3/26/99)
Popular Version of Paper
FC19.05
Monday afternoon, March 22, 15:27 EST
APS Centennial Meeting, Atlanta
We are able to see because a molecule in our eyes (rhodopsin) changes shape reliably when it absorbs visible light. The new shape is detected by the rod or cone cells and reported as a nerve signal. Vitamin A becomes the optically-reactive part of rhodopsin. Deficiency of this vitamin results in night blindness, a fact perverted into a wives tale about carrots improving vision.
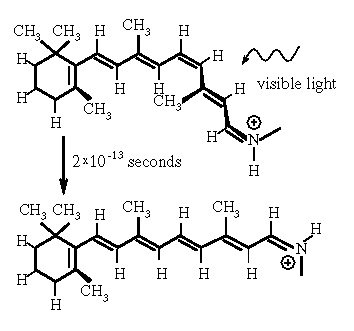
The speed of the photochemical reaction is astounding: the molecular shape changes in 0.2 trillionths of a second, less than the time it takes light to cross the width of a hair. This light-induced shape-changing reaction was discovered 40 years ago and has since been probed by faster and faster lasers. However, there was not an adequate explanation of why the reaction works so well and so fast.
In order to understand the dynamics of optically-active molecules like rhodopsin (a family of molecules called "conjugated polyenes"), we have developed a model which retains the crucial quantum mechanics and yet is still computationally feasible. Our quantum computation for rhodopsin requires solving a matrix with more than 100 trillion (1014 ) numbers thousands of times. This feat is just within the realm of possibility using a powerful computer and some clever numerical methods. To our great delight, emerging from all these powerful methods is a remarkably elegant and intuitive understanding of why a photon makes these molecules change shape.
Simply put, the molecular shape is determined by competing forces. The double bonds are a stabilizing influence, trying to keep the molecule flat, in a plane. Double bonds are depicted in the image as parallel lines. Short-range repulsive forces arise from neighboring atoms squeezing too closely together; these so-called "steric" interactions destabilize the flat state in preference of a three-dimensional shape. The force due to the double bonds ordinarily dominates, leading to the flat resting state of the molecule. Nevertheless, the contribution from steric interactions can still be observed in measurements of molecular vibrations which indicate less stabilization than double bonding forces alone.
When a molecule absorbs a photon, the double bonds are disrupted. Blam! The destabilizing steric interactions become dominant and the molecule responds by twisting. We have calculated the twisting rates for some smaller and simpler chemical relatives of rhodopsin, and found that they agree well with observations. (The article describing this work has been submitted but not yet accepted for publication.) Our next step is to apply our model and methods to calculations on larger molecules.
This work was done in collaboration with Brian Gerke (Williams College), Lucas du Croo de Jongh (Leiden University), and Wim van Saarloos (Leiden University).